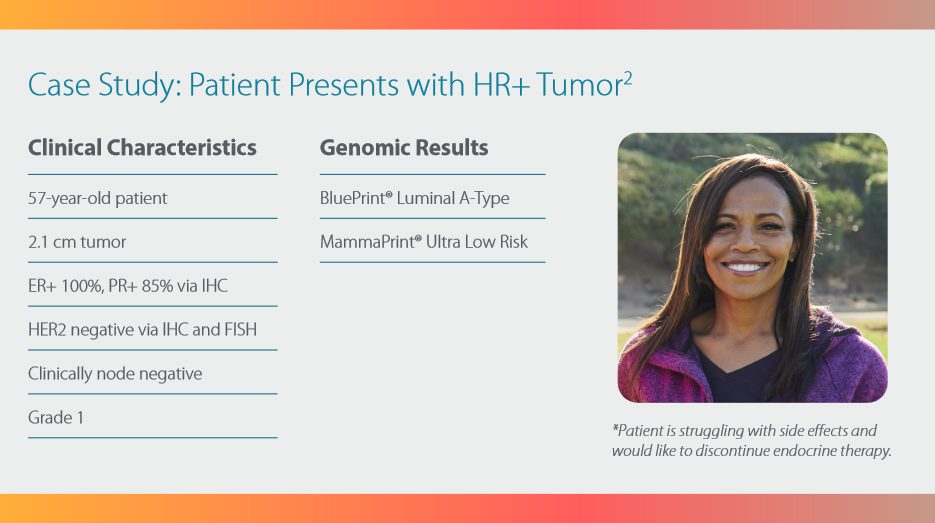
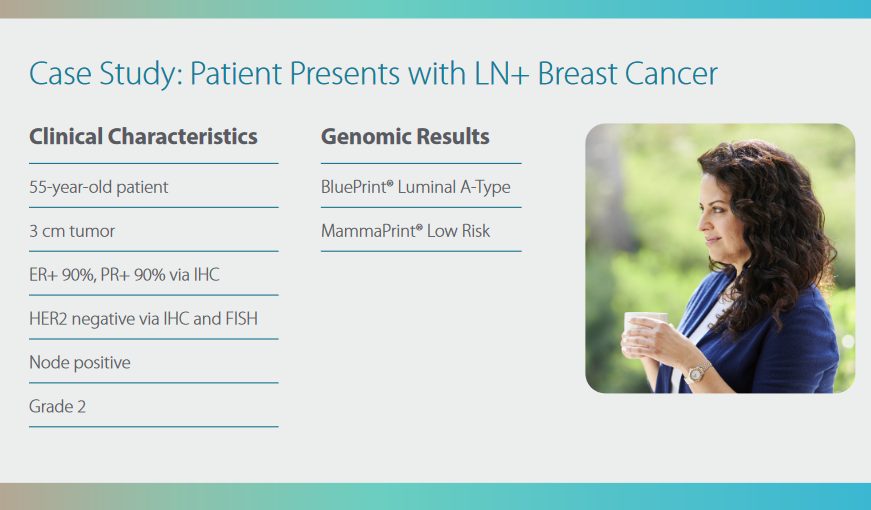
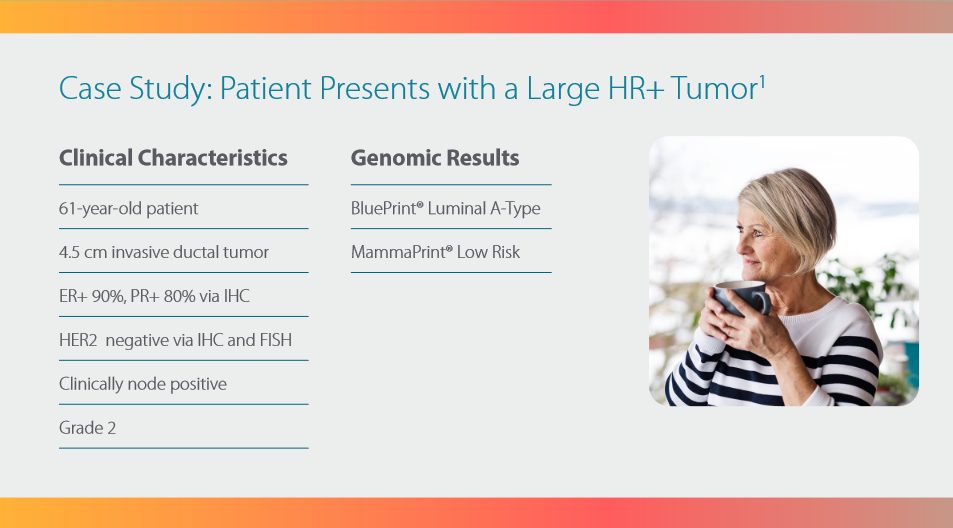
The MammaPrint and BluePrint
screen provides a clear answer
to personalize the treatment for
early-stage hormone receptor
positive breast cancer with
up to three (3) nodes.
MammaPrint is done on FFPE
blocks and identifies genetic
low risk individuals who can
safely forgo chemotherapy.
BluePrint screens the tumor for
80 additional genes Oncologists
can use to tailor the endocrine
therapies for the best outcome.
Did you know? Before genetic
testing treatment plans were only
based on physical things we can see.
At the genomic level physicians can
now examine the tumor activity to
make a more informed decision.
The MammaPrint is FDA-cleared
and backed by peer-reviewed data
with the highest level A1 evidence.
MammaPrint is reimbursed by many
medical funders in Southern Africa.